Sun, Jul 20, 2025
[Archive]
Volume 16, Issue 4 (December 2019)
IJMSE 2019, 16(4): 43-52 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Abbasian A R, Rahimipour M R, Hamnabard Z. Sintering Behavior of Lithium Meta Titanate Nanocrystallites. IJMSE 2019; 16 (4) :43-52
URL: http://ijmse.iust.ac.ir/article-1-1208-en.html
URL: http://ijmse.iust.ac.ir/article-1-1208-en.html
Abstract: (23735 Views)
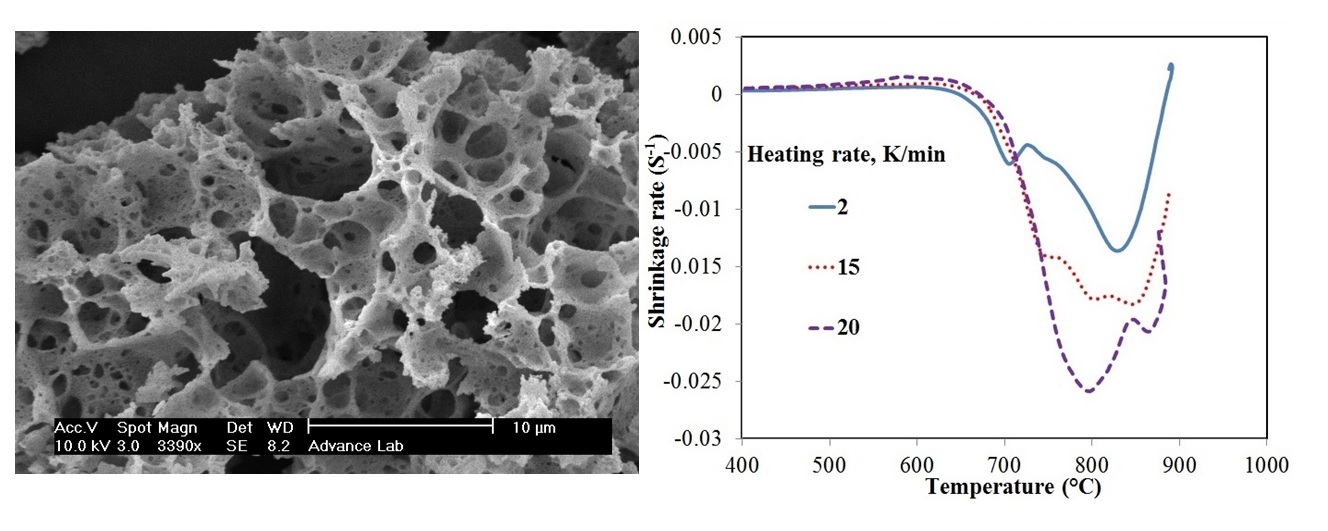
In this work, lithium meta titanate (Li2TiO3) nanocrystallites were synthesized by hydrothermal method and subsequent heat treatment. The shrinkage of the powder compact was measured under constant heating rate in order to study the sintering behavior of the synthesized powders. Densification curves of the synthesized powders were also constructed via the dilatometry analysis and evaluated at several heating rates. Two separate methods of analytical procedure and master curve sintering were employed to determine the activation energy of the initial sintering stage. The activation energy values were estimated based on these two distinct methods as 229±14 and 230 kJ/mol respectively, consistenting with each other. Moreover, surface diffusion was determined as the dominant mechanism of densification on initial sintering of Li2TiO3 nanocrystallites.
Keywords: Tritium breeding, Sintering, Activation energy, Nano, dilatometry, Li2TiO3 nanocrystallites
Type of Study: Research Paper |
Subject:
Ceramic Materials and Engineering
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |